Smitha Padmanabhan1*, T.R Srinivasan1, Narayanasany Kannan1
1 Department of Biotechnology, KSR College of Technology, Tamilnadu, India
*Corresponding Author: Smitha Padmanabhan, M.Sc. Email: [email protected]
DOI: http://dx.doi.org/10.14206/canad.j.clin.nutr.2014.01.05
ABSTRACT
Background: Endosulfan is a neurotoxic organochlorine insecticide, which is used worldwide for pest control and its residues have been remained for long periods in soil, water bodies and agricultural products. Objective: The aim of the present study was to isolate and characterize a bacterium from the earthworm gut which can degrade endosulfan without producing any toxic metabolites like endosulfan sulphate. Methods: Endosulfan was used as a carbon source by the bacteria and degraded it up to 90% within 15 days. The bacteria grew well in minimal medium with high concentrations of endosulfan (80µg/ml). Results: Almost complete degradation of the endosulfan insecticide was indicated by an increase in chloride ion in the growth medium. This strain was able to survive well at 45ºC and exhibited its capability for degradation of the pesticide. Conclusion: The results suggest that the isolated strain is valuable for bioremediation of endosulfan-contaminated soil and water bodies.
Full Text
INTRODUCTION
Endosulfan is a neurotoxic organochlorine insecticide of the cyclodiene family of pesticides. It is highly toxic and an endocrine disruptor, it is toxic to fish and aquatic invertebrates (1). Hans et al., studied the toxicity of endosulfan to earthworm metaphire posthuma and found that the symptoms of toxicity were dose dependent (2). And it is banned in the European Union, the Philippines, Cambodia, and several other countries. It is still used extensively in many countries including the US and India. Endosulfan has been used in agriculture around the world to control insect pests including whiteflies, aphids, leafhoppers, Colorado potato beetles, cabbage worms, and other pests. In India, more endosulfan is used than any other pesticide except mancozeb and monocrotophos, with almost 180 million pounds manufactured in the period 1999-2000. Because of its unique mode of action, it is useful in resistance management. In 2001, in Kerala, India, endosulfan spraying became suspect when linked to a series of abnormalities noted in local children. Initially endosulfan was banned, yet under pressure from the pesticide industry this ban was largely revoked. In 2006, in Kerala, compensation of Rs 50,000 was paid to the next kin of each of 135 people who were identified as having died as a result of endosulfan use. These environmental and health concerns have led to an interest in post-application detoxification of the insecticide.
Endosulfan contamination has been detected in soil, water, air, and food products because of its abundant usage and potential for environmental transport (3, 4). Some bacterial and fungal strains that degrade endosulfan isomers in liquid culture have been isolated and characterized in many laboratories (5, 6). Some strains from soil were also found capable of degrading chlorinated hydrocarbon pesticides (7). The majority of the endosulfan biodegradation studies reported the formation of endosulfan sulphate (8, 9). The degradation product endosulfan sulfate has been found toxic to biomolecules isolated a soil bacteria Klebsiella pneumoniae KE-1 that degrades endosulfan without formation of the toxic metabolite endosulfan sulfate (10, 11).
Earthworms are often exposed to various pesticides and other organic pollutants in the soil and their intestines have excellent detoxification capability with a large number of aerobic and anaerobic bacteria (12), but their use in bioremediation of endosulfan-contaminated soil has so far not been studied. Endosulfan degradation by Rhodococcus isolated from earthworm gut also reported for its degradation potential without producing any toxic metabolites (13). The aim of the present study was to isolate the bacteria from the gut of earthworm which can degrade endosulfan very effectively without producing any toxic metabolites like endosulfan sulphate.
METHODS
COLLECTION OF EARTHWORM
The earthworms used in this study were collected from fields having past histories of endosulfan contamination. Mainly these earthworms collected from the places such as Muthalamada mango tree plantations and Attappadi cashew plantations. Mature and healthy earthworms of equal size were stored under laboratory conditions for 1 week. Further, they were treated with 1µg/g of technical endosulfan in soil by the soil pot method (2).Then these pots were exposed to specific environmental conditions such as temperature and humidity.
ISOLATION OF THE ENDOSULFAN DEGRADING BACTERIA
The earthworms were then dissected, the gut was aseptically removed (14) and homogenized in phosphate-buffered saline (pH 7.0), and the gut flora were cultured in minimal media (MM) containing 0.1 µg/mL endosulfan as the only carbon source. The isolated bacteria from the earthworm gut were then purified on nutrient agar, (pH 6.5) plated in duplicate, and incubated at room temperature (30ºC) for 48 h. The isolated colonies were picked and streaked on nutrient agar to obtain the pure culture.
CALCULATION OF CFU’S
The bacterial strains isolated from the pesticide treated earthworms were grown on nutrient agar and in nutrient broth with different concentrations (0.1, 1.0, 10.0, and 100 µg/mL) of endosulfan to determine the highest concentration at which the strains can survive. Optical density (OD) was measured at 560 nm in nutrient broth and colony-forming units (CFU) on agar plates. Increase in optical density was observed up to 10 µg/mL endosulfan. The bacteria growing at 10 µg/mL endosulfan were isolated and cultured in minimal media plates with multiple concentrations of endosulfan (10, 20, 30, 40, 50, 60, 70, 80, and 90 µg/mL). At all the concentrations of endosulfan up to 80 µg/ml, growth was observed. At the highest concentration (80 µg/ml), one strain was found growing.
GRAM STAINING
The gram staining has been done for the isolated bacteria to identify whether the bacteria belong to gram positive or gram negative groups.
THERMAL STABILITY TEST
The strain was streaked onto a nutrient agar plate and its sensitivity toward increased temperature was studied by keeping it in an incubator at different temperature ranging from 10 to 60ºC.
DETERMINATION OF PHOTO STABILITY OF THE ORGANISM
The photo stability of the organism was checked under exposure to sunlight for 2 h. The photo stability of the isolated bacterium was studied at 5th, 10th, and 15th day.
BIOCHEMICAL CHARACTERIZATION
Biochemical characterization of the isolated bacterium has been done using the IMVIC tests. The IMVIC tests consist of four different tests include indole production, Methyl red, Voges –Proskaur and Citrate utilization.
THIN LAYER CHROMATOGRAPHY
The degradation of endosulfan, with parallel uninoculated controls, was monitored by thin-layer chromatography (TLC) and gas chromatography (GC). The TLC was developed in hexane: chloroform: acetone (9:3:1), on silica gel 60 G plates, and separated spots were analyzed by AgNO3–chromogenic reagent.
CHLORIDE ION ESTIMATION
Degradation of the endosulfan and bacterial growth were occurred simultaneously and an increase in chloride ion in the growth medium was noted, indicating almost complete degradation of the insecticide. The chloride content in the culture medium containing endosulfan was estimated calorimetrically at 0, 5, 10, and 15 days (15), after the incubation of bacteria. The 1.5 ml aliquots were drawn from the flasks. The cells were washed thoroughly by centrifuge (10,000 rpm for 10 min) to remove loosely bound pesticide. The supernatants were pooled which was then used for chloride ion estimation.
PLASMID DNA ISOLATION
Bacterial culture was grown in nutrient broth. The overnight culture was centrifuged at 10000 rpm for 10-15 minutes. To the pellet, 100 µl of ice cold solution I (TEG) was added and vortexed for a few seconds. 200 µl of solution II (10N NaOH) was added, mixed thoroughly and stored in ice for 5 minutes. 150 µl of ice cold solution III (Potassium acetate (3M)) was added, vortexed for 10 seconds, the tubes were stored in ice for 3-5 minutes. This was then centrifuged at 13,000 rpm for 15 minutes. Equal volumes of phenol:chloroform:isoamylalcohol was added to the supernatant and vortexed. The contents were centrifuged for 10,000 rpm for 5 minutes. Ice cold 70% ethanol was added to the supernatant and kept in -20ºC overnight. This was centrifuged at 10,000 rpm for 15 minutes. Supernatant was discarded and the tube was allowed to air-dry. The pellet was suspended in 10 µl TE buffer. The sample was electrophoresed in 0.7% agarose gel.
RESULTS
As shown in figure 1, most viable bacteria were isolated from the guts of earthworms collected from endosulfan-contaminated soil. Survival of the bacterial strain was studied successively in different concentrations of endosulfan. One strain was found to tolerate 80 µg/ml concentration of endosulfan. The isolated organism was found to be Gram positive. On observation under the microscope the cells are seen as violet rods. At 10, 20, 30, 40, 50 and 60 ºC, the thermal stability of the isolated bacterium was studied. Significant growth between 10 and 45C was noted for the isolated bacterium growing at 80 µg/ml. The same strain can survive at 45ºC. The strain survives well under exposure to sunlight.
The results described in table 2 showed that, the calculated CFU results were described in table 1. Biochemical tests were performed according to “Bergey’s Manual of Systematic Bacteriology”, for identification of the bacterial strains. Based on morphological, cultural and biochemical characteristics, the isolated bacterium can be characterized. Methyl red sowed high dense red color which indicates the positive reaction. Catalase (CAT) production with high effervescence when added with hydrogen peroxide. The citrate utilization test also found to be positive. Voges proskauer, indole production, and oxidase production were found to be negative.
α and β isomers were almost totally degraded with some residue of endosulfan diol in 15 days, when the qualitative analysis of the biodegradation product of endosulfan was carried out through thin-layer chromatography. Rate of degradation of endosulfan in the medium was of exponential nature and characterized by fast degradation at the initial stage followed by slow degradation when the degradation in the control flask (UI) was found to be very slow. The results explained in figure 2.
Figure 3 showed that the degradation of endosulfan was characterized by the release of the free chloride ions during the incubation study. This increase in the chloride ions indicates the degradation of endosulfan. The chloride ions produced led to the formation of HCl and thus decrease in the pH of culture medium. Degradation of the endosulfan and bacterial growth occurred simultaneously and an increase in chloride ion (87.1%) in the growth medium, indicating almost completes degradation of the insecticide. Plasmid DNA is isolated from the isolated endosulfan degrading bacteria and it’s subjected to agarose gel electrophoresis for the visualization of the DNA bands. The bands are appeared in orange colour while observing under ultra violet trans-illuminator. The isolated plasmid can be the responsible factor for the degradation of endosulfan (fig 4).
DISCUSSION
The current technique of culturing the most viable bacteria from earthworm gut microflora for endosulfan degradation is a simple and cost-effective one. Successful growth of this strain was observed in a minimal medium containing endosulfan (80 µg/mL) and survives well up to temperature of 45 ºC and under sunlight exposure. Both α and β isomers of endosulfan was degraded by the isolated bacteria. It is to be noted that, like other soil bacteria known for endosulfan degradation (6), it did not produce persistent and toxic metabolite endosulfan sulphate.
The degradation of endosulfan accompanied with formation of endosulfan diol was also found by Awasthi et al. (7) where two Bacillus strains isolated from soil degrade both the isomers of endosulfan by the formation of endosulfan diol and endosulfan lactone. (11) Identified a soil strain Klebsiella pneumoniae KE-1 which degraded endosulfan without forming toxic metabolite endosulfan sulfate. Endosulfan degradation by Rhodococcus isolated from earthworm gut also reported for its degradation potential without producing any toxic metabolites (13). Degradation of endosulfan is clearly indicated by the significant increase of free chloride from endosulfan in medium.
The chloride ions produced led to the formation of HCl and reduction of the pH of culture medium (10). Also documented that the fungal and bacterial strains growing on endosulfan significantly decrease the pH of nutrient culture medium and thus the chance of chemical hydrolysis of endosulfan to endosulfan diol is minimized. α and β isomers were almost totally degraded with some residue of endosulfan diol in 15 days, when the qualitative analysis of the biodegradation product of endosulfan was carried out through thin-layer chromatography. We hypothesize that the isolated plasmid can be the responsible factor for the degradation of endosulfan. We assume that the isolated bacteria degrade endosulfan but are not capable or not efficient at utilizing endosulfan diol as the only source of carbon since it is unable to transport it into the cell. Endosulfan degradation under low-oxygen or anaerobic environments metabolized into endosulfan diol (16), but the strain formed endosulfan diol and degrades it under aerobic conditions without forming endosulfan sulfate.
CONCLUSION
It is concluded that this bacterium will be perhaps more suitable for bioremediation of endosulfan-contaminated Soil, since this bacterium survives up to 45 ºC under intense sunlight. It can also be utilized for bioremediation of endosulfan contaminated water bodies.
ACKNOWLEDGEMENT
First and foremost I would like to express my heartfelt thanks to Lion Dr. K.S. Rangasamy, MJF, my college chairman for providing us with the necessary facilities required for completion of this project. The authors acknowledge the assistance of all the faculty members and technicians in the department of biotechnology for their valuable support and encouragement.
REFERENCES
- Sunderam RIM, Cheng DMH, Thompson GB, Toxicity of endosulfan to native and introduced fish in Australia. Environ Toxicol Chem 1992; 11, 1469–1476.
- Hans RK, Gupta RC, Beg MU, Toxicity assessment of four insecticides to earthworm Pheretima posthuma. Bull Environ Contam Toxicol 1990; 45, 350–364.
- Mansingh A, Wilson A, Insecticide contamination of Jamaican environment. Baseline studies on the status of insecticidal pollution of Kingston Harbour Mar Pollut Bull. 1995; 30, 640–645.
- Miles CJ, Pfeuffer RJ, Pesticides in canals of South Florida Arch Environ Contam Toxicol 1997; 32, 337–345.
- Kullman SW, Matsumura F, Metabolic pathway utilized by Phanerochete chrysoporium for degradation of the cyclodiene pesticide Endosulfan. Appl Environ Microbiol 1996; 62, 593–600.
- Sutherland TD, Horne I, Lacey MJ, Harcourt RL, Russell RJ, Oakeshott JG, Enrichment of an endosulfan – degrading mixed bacterial culture. Appl Environ Microbiol 2000; 66, 2822–2828.
- Awasthi N, Singh AK, Jain RK, Khangrot BS, Kumar A, Degradation and detoxification of endosulfan isomers by a defined co-culture of two Bacillus strains. Appl Microbiol Biotechnol 2003; 62, 279–283.
- Stewart D K R, Cairns KG, Endosulfan persistence in soil and uptake by potato tubers. J Agric Food Chem 1974; 22, 984–986.
- Rao D M R, Murthy A S, Persistence of endosulfan in soils. J Agric Food Chem 1980; 28, 1099–1101
- Siddique T, Okeke BC, Arshad M, Frankenberger Jr WT, Enrichment and isolation of endosulfan degrading microorganisms. J Environ Qual 2003; 32, 47–54.
- Kwon GS, Kim JE, Kim TK, Sohn HV, Koh SC, Shin KS, Kim DG, Klebsiella pnuemoniae KE-1 degrades endosulfan without formation of the toxic metabolite endosulfan metabolite. FEMS Microbiol 2002; Lett. 215, 255–259.
- Karsten G R, Drake HL, Comparative assessment of the aerobic and anaerobic microfloras of Earthworm Guts and Forest Soils. Appl. Environ. Microbiol. 1995; 61, 1039–1044.
- K Verma, N Agrawal, M Farooq, RB Misra, RK Hans, Endosulfan degradation by a Rhodococcus strain isolated from earthworm gut. Ecotoxicology and Environmental Safety 2006; 64, 377–381.
- Ramteke PW, Hans RK, Isolation of hexachlorocyclohexane (HCH) degrading microorganisms from earthworm gut. J Environ Sci Health. 1992; A 27, 2113–2122.
- Bergman J G, Sanik Jr J, Determination of trace amounts of chlorine in naphtha. Anal Chem 1957; 29, 241–243.
- Guerin T F, The anaerobic degradation of endosulfan by indigenous microorganism from low-oxygen soils and sediments. Environ Pollut 1999; 106, 13–21.
Table 1: Calculation of colony forming units
| S.No. | Days | CFU/ml (on agar plates) | Optical density at 560 nm (in nutrient broth) |
| 1. | 1 | 0.86 ´107 | 0.01 |
| 2. | 3 | 1.0 ´107 | 0.06 |
| 3. | 5 | 1.2 ´107 | 0.09 |
| 4. | 7 | 2.0 ´107 | 0.12 |
| 5. | 10 | 5.0 ´107 | 0.181 |
| 6. | 12 | 5.5 ´107 | 0.182 |
| 7. | 15 | 6.0 ´ 107 | 0.183 |
Table 2: Biochemical characterizations
| S.No. | TEST | RESULT |
| 1. | Gram staining | Positive rods or cocci |
| 2. | Indole production | Negative
|
| 3. | Methyl red
|
Positive |
| 4. | Voges -proskauer | Negative
|
| 5. | Citrate utilization
|
Positive |
| 6. | Catalase production
|
Positive |
| 7. | Oxidase production
|
Negative |
| 8.
|
Gelatin hydrolysis | Negative |
Figure 1: Isolated Endosulfan Degrading Bacteria
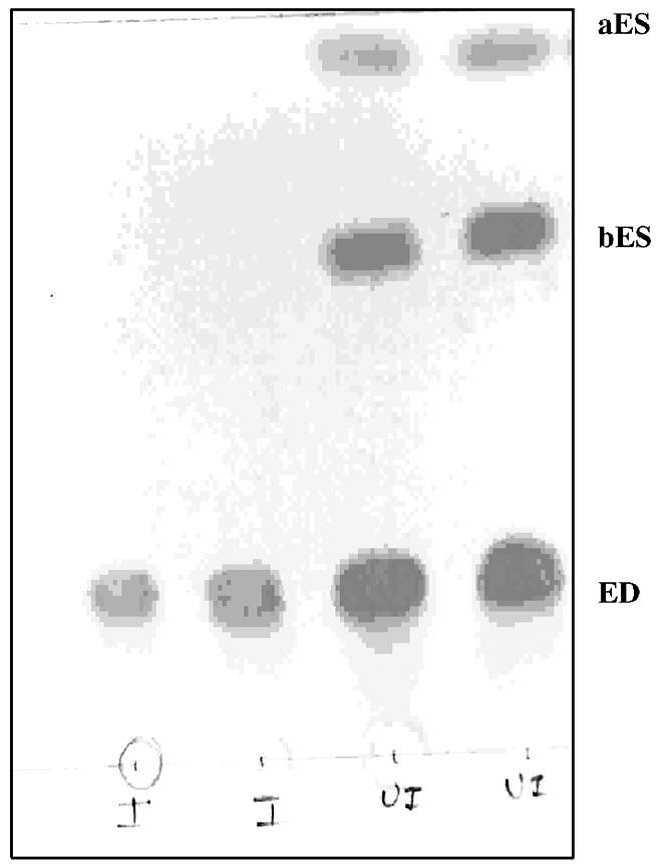
Figure 2: Thin-layer chromatogram showing the degradation of endosulfan during 15 days incubation; aES, α-endosulfan; bES, β endosulfan; ED, endosulfan diol; UI, uninoculated; I, inoculated.
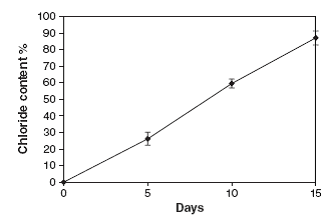
Figure 3: Release of Free Chloride Ions in 15 Days of Incubation
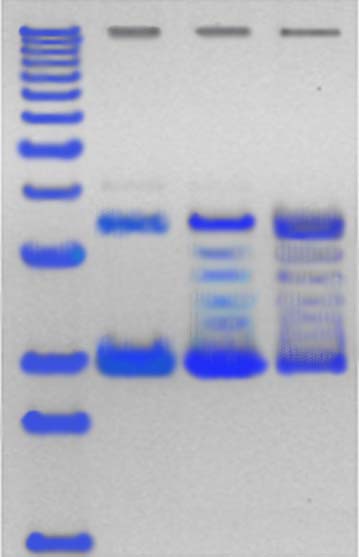
Figure 4: DNA Marker Isolated Bacterium
